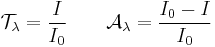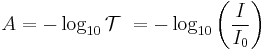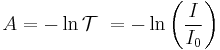Transmittance
In optics and spectroscopy, transmittance is the fraction of incident light (or other electromagnetic radiation) at a specified wavelength that passes through a sample. A related term is absorptance, or absorption factor, [1][2] which is the fraction of radiation absorbed by a sample at a specified wavelength. Occasionally one also hears the terms visible transmittance (VT) and visible absorptance (VA), which are the respective fractions for the spectrum of light visible radiation. In equation form,
where 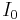 is the intensity of the incident radiation and I is the intensity of the radiation coming out of the sample and
is the intensity of the incident radiation and I is the intensity of the radiation coming out of the sample and 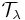 and
and 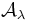 are transmittance and absorptance respectively. In these equations, scattering and reflection are considered to be close to zero or otherwise accounted for. The transmittance of a sample is sometimes given as a percentage.
are transmittance and absorptance respectively. In these equations, scattering and reflection are considered to be close to zero or otherwise accounted for. The transmittance of a sample is sometimes given as a percentage.
For liquids, transmittance is related to absorbance A (not to be confused with absorptance) as
In the case of gases it is customary to use natural logarithms instead, making absorbance A for gases
From the above equation and the Beer-Lambert law, the transmittance for gases is thus given by
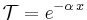 ,
,
where  is the attenuation coefficient and
is the attenuation coefficient and 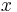 is the path length. For liquids e is replaced by 10.
is the path length. For liquids e is replaced by 10.
Note that the term "transmission" refers to the physical process of radiation passing through a sample, whereas transmittance refers to the mathematical quantity.
References
- ^ "IUPAC handbook definition". http://www.iupac.org/goldbook/A00035.pdf. Retrieved 2009-07-02.
- ^ "CRC Dictionary of pure and applied physics, CRC Press, Editor: Dipak Basu (2001)". http://www.crcpress.com/product/isbn/9780849328909.